

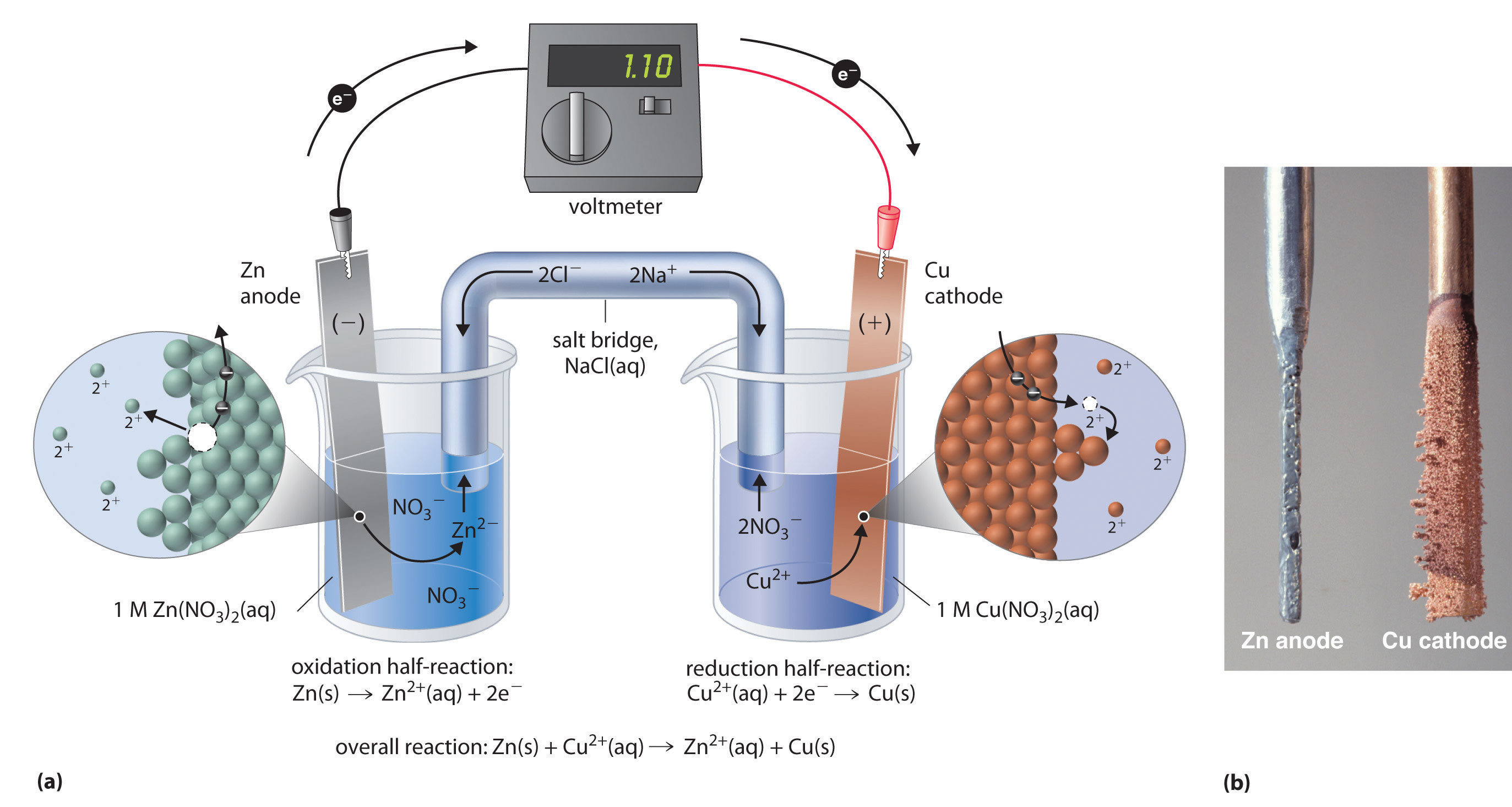
The external circuit provides a conductivity path for Lithium ions and electrons produced during the reaction. The electrolyte of the Lithium-ion battery is a combination of Lithium salts. Lithium-ions and the electrons are transmitted through the electrolytes which then reunite at the cathode in a reduction reaction. During the discharge phase of the battery, an oxidation reaction occurs at the anode which produces Lithium ions (positive), electrons (negative), and some by-products at the anode. The anode, stereotypically, is made up of carbon. The cathode of Lithium-ion batteries is made up of an interpolated Lithium compound, Lithium Manganese Dioxide. Whereas, during the charging process of the Lithium-ion battery ions travel from the cathode (positive electrode) to the anode (negative electrode). An exchange of ions occurs between the anode and the cathode with the help of material in between, the electrolyte.ĭuring the discharging state of the battery, lithium ions travel from the anode (negative electrode) to the cathode (positive electrode) via an electrolyte. Just like any other electrolytic reaction, the reaction inside the Lithium-ion battery is the same. How do Anode and Cathode Work In Lithium-ion Batteries Cathode and anode play a major part in the chemical reactions that produce an electrical output. The cathode is a metal oxide and the anode is made up of carbon or graphite. Batteries consist of two parts namely: Cathode and Anode. Batteries are electrochemical devices that make use of chemistry to generate electrical energy. These batteries control almost all aspects of our daily lives and there are different types of them playing their part. In this fast-growing age of technology, surrounded by electronic appliances and equipment, batteries have become very important.


 0 kommentar(er)
0 kommentar(er)
